We are your Life Sciences Experts
See our Life Sciences ExpertiseBusiness & Decision is hiring
Apply nowBusiness & Decision Life Sciences joined the Orange Group
Orange Healtcare websiteOur CRO as partner in your digital transformation
See our Digital & Data ExpertiseA Human-centric, Agile, Innovative and Collaborative Company
Join us
From strategic consulting to delivery, our Engineers, Clinicians, Pharmacists and Data Specialists are here to support you in all your projects of Clinical Trials, Biometrics, Pharmacovigilance, Regulatory and Quality Compliance.
As the CRO of the Orange Group and Digital Transformation partner, our multidisciplinary teams will support you throughout the whole journey of your clinical data (collection, transport, security and hosting), and in their processing thanks to an optimal combination of Big Data, Data Sciences (AI), and the professional added value of our Life Sciences experts.
B&D Life Sciences is the only company that can bring you the entire value chain needed for digitalizing the observance of patients both in real life, as in clinical trials.
Have a look the interview of Yoann COTTE to learn more
25 years of experience
moving your project forward
In The News
-
 LinkedIn04Nov2022
LinkedIn04Nov2022PHUSE EU CONNECT 2022
We will be present at the PHUSE EU Connect 2022 held in Belfast from 13th to 16th November.
-
 Article17Dec2021
Article17Dec2021We are officially Great Place to Work certified!
Today it is official!
-
 Article01Jun2021
Article01Jun2021Our website has been renewed
You may have noticed our website has changed
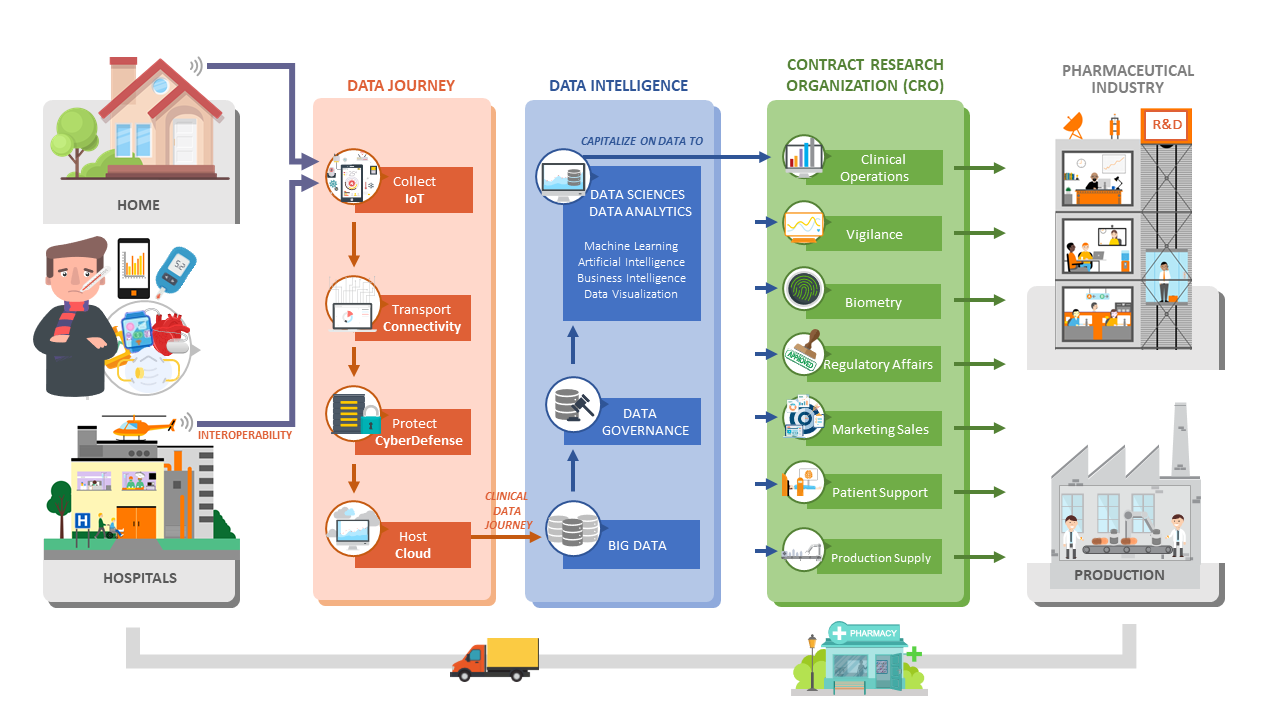
Business & Decision Life Sciences contributes to your digital journey trough Data Governance
Taking advantage of our expertise in Clinical Data Management, Statistics, CDISC data standards and regulation vigilance. Business & Decision Life Sciences contributes to your digital journey trough Data Governance and Data Validation services. Digitalization is the key accelerator to optimize submissions. Data Governance defines an environment structure and maintains processes and roles, ensures control, traceability and transparency, assembles structures and values in CDASH / SDTM / ADaM, facilitates conversion from one standard to the other, ensures consistency and FDA compliance.
Applying Data Governance and using libraries accelerate study set-up. Digitalization is the key accelerator to optimize R&D, makes large and complex data more accessible, enables identification of trends, provides facts and figures, gives real time status, enables follow-up of KPIs, supports decision making. Data Visualization improves study management efficiency and eases regulation submission.
Business & Decision proposes a unique blend of Life Sciences specialists and data capabilities.